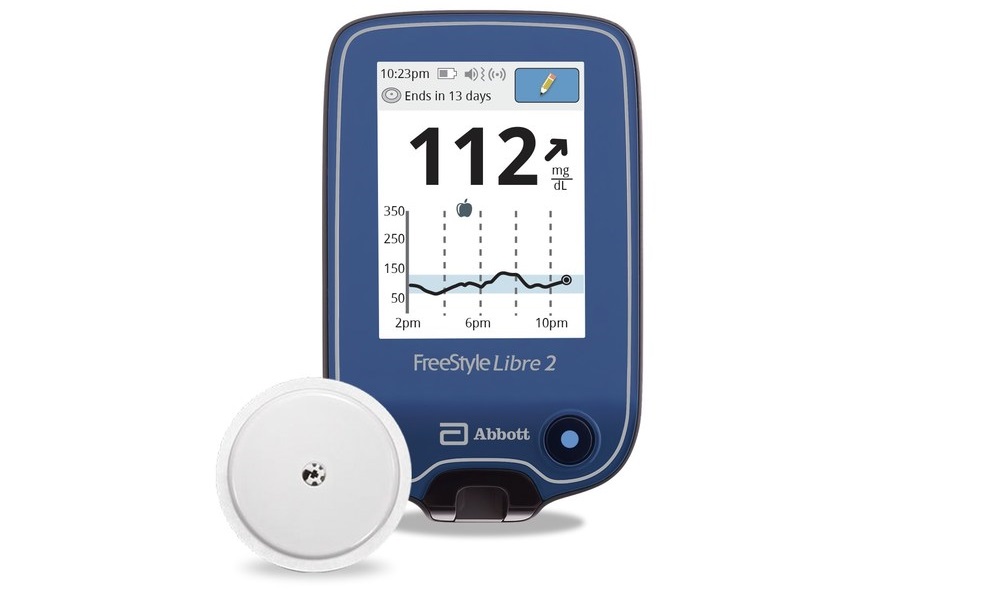
Abbott (Abbott Park, IL) announced that the U.S. Food and Drug Administration (FDA) cleared its next-generation FreeStyle Libre 2 integrated continuous glucose monitoring (iCGM) system for adults and children ages 4 and older with diabetes.
According to the company, this is the only iCGM system with optional real-time alarms that measures glucose levels every minute, meeting the highest level of accuracy standards over 14 days, including superior day one accuracy compared to the other iCGM and excellent accuracy and alarm performance at low end glucose levels.
With a 14-day wear time, the FreeStyle Libre 2 system is the longest-lasting, self-applied iCGM sensor currently available, eliminating the need for fingersticks – and priced at a third of the cost of other CGM systems. Using Bluetooth technology, the FreeStyle Libre 2 system automatically alerts users when their glucose is high or low without needing to scan the sensor. Users also have the option of turning off the customizable, real-time alarms.