September 21, 2023- Medtronic, a global leader in healthcare technology, announced CE (Conformité Européenne) Mark approval for its new all-in-one, disposable Simplera™ continuous glucose monitor (CGM) featuring a simple, two-step insertion process.
Simplera™ is indicated for ages 2+ and compatible with iOS and Android. Simplera™ is not approved by the FDA and is limited to investigational use in the U.S. Medtronic’s automated insulin delivery (AID) system integrated with this next-generation sensor is currently under review for CE Mark and is not commercially available in the U.S. or in Europe.
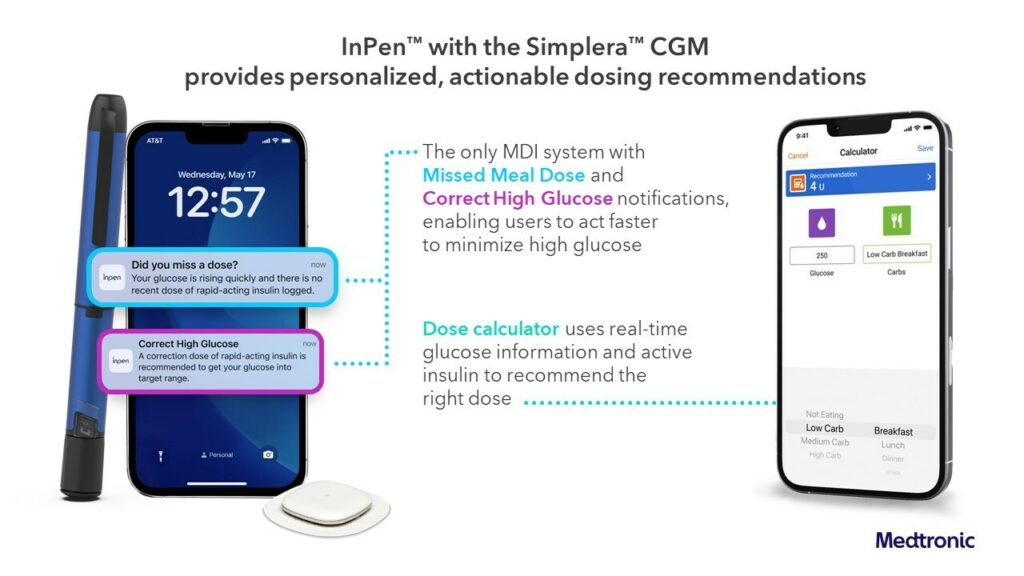
The company’s newest no-fingerstick sensor does not require over tape and is seamlessly integrated with the InPen™ smart insulin pen, which provides real-time, personalized dosing guidance to help simplify diabetes management.