September 4, 2020 - Roche (Basel, Switzerland) announced that the cobas SARS-CoV-2 & Influenza A/B Test for use on the cobas 6800/8800 Systems received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA). This test is intended for the simultaneous qualitative detection and differentiation of SARS-CoV-2, Influenza A, and Influenza B in … [Read more...]
B. Braun receives FDA clearance for SpaceStation MRI
August 31, 2020 - B. Braun Medical Inc. (Bethlehem, PA) received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the SpaceStation MRI to allow Space® infusion pumps to continuously deliver medications to patients within the MRI suite. The SpaceStation MRI is designed to shield Space infusion pumps against 1.5-T and 3.0-T magnetic fields to protect the … [Read more...]
Roche receives FDA approval of FoundationOne Liquid CDx, comprehensive pan-tumor liquid biopsy test
August 28, 2020 - Roche (Basel, Switzerland) received U.S. Food and Drug Administration (FDA) approval for FoundationOne Liquid CDx, Foundation Medicine’s comprehensive pan-tumor liquid biopsy test for patients with solid tumors. FoundationOne Liquid CDx is a comprehensive genomic profiling (CGP) test that analyzes more than 300 cancer-related genes and multiple genomic … [Read more...]
Abbott releases fast, $5 COVID-19 antigen test, free mobile app to display test results
August 27, 2020 - Abbott (Abbott Park, IL) has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for its BinaxNOW COVID-19 Ag Card rapid test for detection of COVID-19 infection. The company says it will sell this test for $5. Currently, AdvaMed (The Advanced Medical Technology Association) estimates that test manufacturers are … [Read more...]
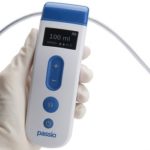
Bearpac Medical launches new Passio Pump Drainage System
August 26, 2020 - Bearpac Medical, LLC (Moultonborough, NH) announced the launch of a new FDA 510(k) cleared device intended to treat patients with malignant and other recurrent pleural effusions. The Passio Pump Drainage System consists of the Passio Catheter, a Handheld Control Unit (a Class II electronic handheld pump) and a Disposable Collection Kit, which … [Read more...]
Philips, B. Braun receive FDA clearance for Onvision Needle Tip Tracking technology for regional anesthesia
August 26, 2020 - Royal Philips and B. Braun Medical Inc. (Bethlehem, PA) announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Onvision, a breakthrough ultrasound guidance solution for real-time needle tip tracking. Available exclusively on the latest version of the B. Braun and Philips Xperius ultrasound system together with the dedicated … [Read more...]
Quidel to update packaging of POC Sofia SARS Antigen test for COVID-19 to include either nasal or nasopharyngeal swabs
August 20, 2020 - Quidel Corporation announecd that labeling for Quidel’s Emergency Use Authorization (EUA) for the Sofia SARS Antigen FIA has been amended to include either nasal or nasopharyngeal swabs. The new kit labeling, with the addition of a nasopharyngeal swab, allows Quidel to offer a second kit configuration to support the nasopharyngeal sample … [Read more...]
BD Veritor System for COVID-19 testing now available
From the upcoming September edition of Repertoire Magazine. August 20, 2020 - Designed to meet the evolving and critical needs of point-of-care (POC) testing, the BD Veritor Plus system enables you to streamline the testing experience. Be ready for flu season by testing with CLIA-waived Flu A+B, Group A Strep, and respiratory syncytial virus (RSV) assays along with the … [Read more...]
Masimo PVi receives FDA clearance as indicator of fluid responsiveness on mechanically ventilated patients
August 17, 2020 - Masimo (Irvine, CA) announced that PVi (pleth variability index) has received FDA clearance as a continuous, noninvasive, dynamic indicator of fluid responsiveness in select populations of mechanically ventilated adult patients. PVi, is a measure of the dynamic changes in perfusion index that occur during the respiratory cycle. Available alongside Masimo … [Read more...]
FDA approves Roche’s Evrysdi (risdiplam) for treatment of spinal muscular atrophy (SMA) in adults, children
August 10, 2020 - Roche (Basel, Switzerland) received approval from the U.S. Food and Drug Administration (FDA) for Evrysdi (risdiplam) for the treatment of spinal muscular atrophy (SMA) in adults and children 2 months of age and older. Evrysdi showed clinically-meaningful improvements in motor function across two clinical trials in people with varying ages and levels of … [Read more...]