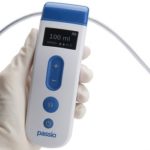
August 26, 2020 - Bearpac Medical, LLC (Moultonborough, NH) announced the launch of a new FDA 510(k) cleared device intended to treat patients with malignant and other recurrent pleural effusions. The Passio Pump Drainage System consists of the Passio Catheter, a Handheld Control Unit (a Class II electronic handheld pump) and a Disposable Collection Kit, which … [Read more...]
Philips, B. Braun receive FDA clearance for Onvision Needle Tip Tracking technology for regional anesthesia
August 26, 2020 - Royal Philips and B. Braun Medical Inc. (Bethlehem, PA) announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Onvision, a breakthrough ultrasound guidance solution for real-time needle tip tracking. Available exclusively on the latest version of the B. Braun and Philips Xperius ultrasound system together with the dedicated … [Read more...]
Quidel to update packaging of POC Sofia SARS Antigen test for COVID-19 to include either nasal or nasopharyngeal swabs
August 20, 2020 - Quidel Corporation announecd that labeling for Quidel’s Emergency Use Authorization (EUA) for the Sofia SARS Antigen FIA has been amended to include either nasal or nasopharyngeal swabs. The new kit labeling, with the addition of a nasopharyngeal swab, allows Quidel to offer a second kit configuration to support the nasopharyngeal sample … [Read more...]
BD Veritor System for COVID-19 testing now available
From the upcoming September edition of Repertoire Magazine. August 20, 2020 - Designed to meet the evolving and critical needs of point-of-care (POC) testing, the BD Veritor Plus system enables you to streamline the testing experience. Be ready for flu season by testing with CLIA-waived Flu A+B, Group A Strep, and respiratory syncytial virus (RSV) assays along with the … [Read more...]
FDA Approves Roche’s ENSPRYNG for Neuromyelitis Optica Spectrum Disorder (NMOSD)
August 17, 2020 - Roche (Basel, Switzerland) announced that the U.S. Food and Drug Administration (FDA) has approved ENSPRYNG (satralizumab-mwge) as the first and only subcutaneous treatment for adults living with anti-aquaporin-4 (AQP4) antibody positive neuromyelitis optica spectrum disorder (NMOSD). NMOSD is a rare, lifelong and debilitating autoimmune disorder of the … [Read more...]
Masimo PVi receives FDA clearance as indicator of fluid responsiveness on mechanically ventilated patients
August 17, 2020 - Masimo (Irvine, CA) announced that PVi (pleth variability index) has received FDA clearance as a continuous, noninvasive, dynamic indicator of fluid responsiveness in select populations of mechanically ventilated adult patients. PVi, is a measure of the dynamic changes in perfusion index that occur during the respiratory cycle. Available alongside Masimo … [Read more...]
OraSure announces progress on new COVID-19 testing products
August 06, 2020 - OraSure (Bethlehem, PA) along with its quarterly financial results, announced what the company is doing to help the industry combat the COVID-19 pandemic. The company says it is well positioned to support multiple modes for COVID-19 testing, including PCR/molecular, antigen, and antibody testing. To support capacity building for the company’s existing … [Read more...]
Roche receives FDA authorization for Epstein-Barr virus quantitative test on the cobas 6800/8800 Systems
August 06, 2020 - Roche (Basel, Switzerland) announced that the U.S. Food and Drug Administration (FDA) has authorized the cobas EBV test. This is the first quantitative in vitro diagnostic test for Epstein-Barr virus (EBV) DNA in the U.S., the company claims. The authorization gives healthcare professionals the ability to run a large number of patient tests for the … [Read more...]
Medtronic announces comprehensive U.S. launch of new InterStim Micro Neurostimulator
August 05, 2020 - Medtronic plc (Dublin, Ireland) announced that its recently FDA-approved InterStim Micro neurostimulator for sacral neuromodulation (SNM) therapy is now available in the U.S. Cleveland Clinic performed the first patient implant in the nation with the new device. At 2.8 cm, the device is 50% smaller than the market’s other rechargeable SNM device, making … [Read more...]
Medtronic receives FDA approval for two new products: InterStim Micro Neurostimulator, InterStim SureScan MRI leads
August 03, 2020 - Medtronic plc (Dublin, Ireland) received approval from the U.S. Food and Drug Administration (FDA) for its InterStim Micro neurostimulator and InterStim SureScan MRI leads. InterStim Micro — the market’s smallest rechargeable device to deliver sacral neuromodulation (SNM) therapy — is used for treating overactive bladder (OAB), fecal incontinence (FI) and … [Read more...]