B. Braun
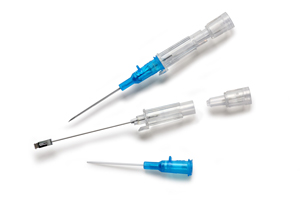 The B. Braun Introcan Safety® family of peripheral IV catheters offers truly passive safety features that are activated automatically and cannot be bypassed. Both the Introcan Safety and the Introcan Safety 3 Closed IV Catheter minimize needlestick injuries and promote first-stick success. The Introcan Safety 3 Closed IV Catheter provides an added safety feature with an automatic, multi-access blood control septum, preventing blood exposure after withdrawing the needle and every time the hub is accessed.
The B. Braun Introcan Safety® family of peripheral IV catheters offers truly passive safety features that are activated automatically and cannot be bypassed. Both the Introcan Safety and the Introcan Safety 3 Closed IV Catheter minimize needlestick injuries and promote first-stick success. The Introcan Safety 3 Closed IV Catheter provides an added safety feature with an automatic, multi-access blood control septum, preventing blood exposure after withdrawing the needle and every time the hub is accessed.
The passive safety shield deploys automatically and stays in place during disposal. Additionally, it encourages best practice by preventing needle reinsertion. The Introcan Safety Family of PIVCs helps facilities cut costs and go green by generating less waste with smaller, lighter components. Your customers will avoid throwing away unused components (compared to integrated catheters) and help cut costs by reducing needlesticks, cleanup time and materials.
Braun Introcan Safety 3’s multiple-access blood control septum reduces exposure to blood when the needle is removed and every time the hub is accessed. Additionally, Introcan Safety 3’s integrated stabilization platform improves catheter stability and minimizes movement within the vessel to help reduce catheter related complications.
Probing sales questions
- “Doctor, has anyone in your facility experienced a needlestick injury?”
- “Does your current IV catheter include an active safety mechanism where the user must manually activate to get the proper protection?”
- “Is there a concern for wasted product?”
- “Is first-stick success important?”
The Introcan Safety IV Catheters’ double flashback technology allows visualization of both needle and catheter vein entry. The initial visual of blood in the flashback chamber indicates needle entry into the vein. A secondary flashback occurs in the catheter as it is advanced, confirming accurate placement and facilitating first stick success.
Final points
Braun provides value-added products with customers and their patients always in mind. In addition to preventing needlestick injuries and facilitating first-stick success, the Introcan Safety IV Catheters are small by design. The smaller, lighter components will save room in sharps containers, generating less waste for the facility and providing additional savings.
Sponsored by B. Braun.
Recent studies1, 2 confirm that passive safety designs offer 2-3 times better protection against accidental needlesticks than active designs. INS Standards also recommend the use of passive safety devices.1 Tosini W, et al. “ Needlestick Injury Rates According to Different Types of Safety Engineered Devices: Results of a French Multicenter Study”, Infection Control and Hospital Epidemiology, April 2010. Vol 31, Number 4
2Shimatani M, et al. “Comparison of the Needlestick Injuries Due to Active and Passive Design Safety Intravenous Catheters”, Poster Presentation APIC 2011. Submitted for publication 2011
Crosstex/SPSmedical
 Healthcare providers throughout the world are steadily recognizing that better awareness and prevention of healthcare associated infections not only saves lives, but ultimately saves money and drives greater efficiencies in the healthcare system. It takes unique expertise, commitment, skills and enhanced products to do this correctly, and growing numbers of providers are devoting additional resources to this important area. Crosstex/SPSmedical seeks to continue developing novel products that address these critical issues as we believe infection prevention and control markets will continue to grow for years to come.
Healthcare providers throughout the world are steadily recognizing that better awareness and prevention of healthcare associated infections not only saves lives, but ultimately saves money and drives greater efficiencies in the healthcare system. It takes unique expertise, commitment, skills and enhanced products to do this correctly, and growing numbers of providers are devoting additional resources to this important area. Crosstex/SPSmedical seeks to continue developing novel products that address these critical issues as we believe infection prevention and control markets will continue to grow for years to come.
Secure Fit® Technology Face Masks from Crosstex/SPSmedical are an example of such a novel product. Secure Fit® Technology Face Masks are proven to provide up to three times greater protection over other face masks*. Secure Fit® Technology Face Masks feature aluminum nose and chin pieces that can be adjusted to fit the shape and size of any face, while significantly reducing the gapping on the sides and bottom of the mask, reducing exposure to airborne particulates and aerosols by more than three times that of a standard earloop face mask. Secure Fit® Technology Face Masks are made in the USA and come in all three ASTM levels. *Study on file
Greater efficiency, lower costs, better outcomes
Secure Fit® Technology Face Masks are considerably less expensive than respirators, provide better breathability, better fit and better feel than that of a surgical tie-on mask, are made in the USA, and are readily available in all three ASTM levels.
Probing sales questions
- “Doctor, what type(s) of exposure do you experience when interacting with patients?”
- “Tell me about the appropriateness of your current masks for these exposures.”
- “What ASTM level is your current decontamination mask?”
- “Do you feel protected when you are wearing your mask?”
Potential objections
“Ear loop face masks do not offer the same level of protection as surgical tie-on masks.”
Rep response: “This is not true. Secure Fit® Technology Face Masks come in ASTM levels 1-3. ASTM level 3 masks provide the highest level of protection from aerosols and fluid sprays. The Secure Fit® technology conforms to any size or shape face giving a custom fit. This reduces gapping around the chin and cheeks for a better fit and increased protection.”
“Our current decontamination mask is on contract and is less expensive.”
Rep response: “Yes, but does it provide the protection needed for staff? What ASTM level is your current mask?” Often, the masks used are minimum performance masks. A Secure Fit mask that meets ASTM level 1-3 criteria will provide the protection needed while cleaning contaminated instruments.
Final points
Crosstex/SPSmedical recognizes that protection, comfort and fit are the most important roles for face masks in the healthcare market. This is why our Secure Fit® Technology Face Masks are made from only the highest quality materials and tested to ensure premium performance, which protects healthcare workers and patients alike.
See how safe really feels: Compare a fitted mask with Secure Fit® Technology to a standard mask. Request a FREE sample of up to four different styles. Visit www.crosstex.com/facemasks or call 800-722-1529.
Sponsored by Crosstex/SPSmedical.
Georgia-Pacific Professional
 Ensuring your clients’ hygiene compliance in their facilities represents a meaningful opportunity not only to advocate for health and hygiene compliance, but also to develop and cultivate your client relationships. Here’s why:
Ensuring your clients’ hygiene compliance in their facilities represents a meaningful opportunity not only to advocate for health and hygiene compliance, but also to develop and cultivate your client relationships. Here’s why:
Did you know that healthcare-associated infections (HAIs) affect 1.7 million patients each year in the United States? That this is 5-10 percent of hospitalizations? That this accounts for about 99,000 deaths? HAIs can be acquired at any healthcare facility, and they add nearly $20 billion to healthcare costs annually, according to the Centers for Disease Control and Prevention.
As healthcare sales professionals and client advocates, you can help your clients prevent HAI transmission. Ensuring that clients follow proper hand hygiene techniques is the most important and least expensive way to do this; but on average only 40 percent of all healthcare workers practice proper hand hygiene.
How do Georgia-Pacific Professional products help promote hygiene and help reduce cross-contamination?
One method to help your clients reduce cross-contamination is to upgrade to touchless dispensers. Using Georgia-Pacific Professional automated no-touch product dispensers, and no-touch towel dispensers for single-use disposable paper towels, helps reduce potential opportunities for cross-contamination. Georgia-Pacific Professional soap and hand sanitizer dispensers also help reduce the risk of cross-contamination with a closed system consisting of the bag, pump and nozzle.
Everyone wins by promoting hygiene: your clients, their patients and employees. By advocating hand hygiene, you are positioning yourself as a resource who will provide meaningful solutions to issues your clients care about in the management of their healthcare facilities, as well as helping them become more hygienic and efficient. In addition to building a deeper client relationship, by selling Georgia-Pacific Professional products, you are also laying the groundwork for continued sales and commissions far into the future.
Sponsored by Georgia-Pacific Professional
GOJO Industries
 The PURELL ES™ Everywhere System is designed to fit where your customers need it. It is the smallest, most versatile and appealing PURELL® System in the market offering unparalleled placement access and customer convenience. The system is designed to fit in places that are unable to accommodate traditional dispensers or where bottles are commonly found. It features a ready-to-install preassembled base, contains almost twice as much product as a standard eight-fluid ounce bottle and allows for easy, at-a-glance monitoring of product level for easy servicing.
The PURELL ES™ Everywhere System is designed to fit where your customers need it. It is the smallest, most versatile and appealing PURELL® System in the market offering unparalleled placement access and customer convenience. The system is designed to fit in places that are unable to accommodate traditional dispensers or where bottles are commonly found. It features a ready-to-install preassembled base, contains almost twice as much product as a standard eight-fluid ounce bottle and allows for easy, at-a-glance monitoring of product level for easy servicing.
“Hand hygiene needs to be accessible,” says Joey Suntken, GOJO North American Acute Care Market Director. “The PURELL ES™ Everywhere System’s sturdy, one-hand dispensing makes hand sanitizing easy and more accessible. Its small size allows your customers to place it in smaller, high-traffic spaces, such as registration and reception areas where other solutions cannot fit, and replace bottles that can get moved or knocked over. And, increasing the accessibility of hand sanitizer can help increase its usage and help reduce the spread of illness-causing germs.”
The PURELL ES™ Everywhere System is ideal for patient care rooms, the nurse’s station, treatment carts, registration areas, emergency medical services, break rooms and offices. It can be placed where it is needed to help keep staff and patients healthy.
Benefits
The PURELL ES™ Everywhere Systems offers these unique benefits:
- Easy set-up. The kit includes a preassembled base and refill cartridge with 3M™ Command™ strips for trouble free placements.
- Easy-change refill cartridges. Refill cartridge securely attaches to the base, which reliably mounts to surfaces.
- Trouble-free performance. Base constructed of robust plastic for long-term reliability and backed by the GOJO Lifetime Guarantee.
- Efficiency. Refill contains almost twice as much product as standard 236 mL PURELL® pump bottle.
- At-a-glance monitoring. Entire refill exposed for at-a-glance monitoring of product level and easy servicing.
- Effectiveness in a single actuation. Delivers an efficacious amount of PURELL with each actuation.
- Easy recognition. Clear, gem-shaped refill cartridge has the familiar look of the PURELL brand everyone knows and trusts.
- Versatile mounting accessories. Wall mount, horizontal surface mount and rail mount accessories allow for innovative placement options almost everywhere sanitizer is needed.
The PURELL ES™ Everywhere System is our latest advancement as part of a complete line of GOJO products and programs that meet the health and well-being needs in healthcare. Learn more at Healthcare.GOJO.com.
Sponsored by GOJO Industries, Inc.
PDI
 Patients with cancer and autoimmune disorders may be prescribed infusion therapies administered via intravenous route. Infusion therapy can take place at a physician’s office, hospital or ambulatory care facility. These patients can have weakened immune systems, and it is critically important to reduce their risk of contracting a healthcare-associated infection (HAI). In addition, outpatient surgery centers are also focused on preventing HAIs as surgical procedures are moving away from the traditional hospital setting.
Patients with cancer and autoimmune disorders may be prescribed infusion therapies administered via intravenous route. Infusion therapy can take place at a physician’s office, hospital or ambulatory care facility. These patients can have weakened immune systems, and it is critically important to reduce their risk of contracting a healthcare-associated infection (HAI). In addition, outpatient surgery centers are also focused on preventing HAIs as surgical procedures are moving away from the traditional hospital setting.
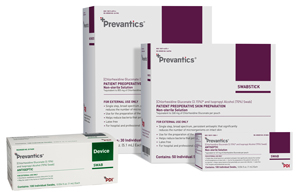
The Prevantics® product line is a comprehensive, evidence-based solution that addresses the vascular access continuum of care. The newest addition, Prevantics® Device Swab:
- Is specifically indicated for the disinfection of needleless sites prior to use.
- Contains the first and only 3.15 percent Chlorhexidine Gluconate and 70 percent isopropyl alcohol formulation.
- Has a quick five-second scrub time and five-second dry time to help with staff compliance.
Greater efficiency
Bloodstream infections continue to be a leading cause of mortality and morbidity for patients with invasive catheters. As physician practices and surgery centers are being purchased by hospital systems, it is becoming increasingly important for these facilities to be compliant with the CDC guidelines for infection prevention in outpatient settings.
By using Prevantics® Skin Antiseptics for pre-injection and pre-operative skin preparation, Prevantics® Device Swab for disinfecting a needleless access site prior to use, practicing hand hygiene, and adhering to aseptic technique when accessing devices, the risk for these serious HAIs can be mitigated.
Probing sales questions
- “What type of skin antiseptic are you using prior to minor surgical procedures or injections?”
- “Do you measure your rates for blood culture contamination and bloodstream infections acquired in your clinic?”
- “If yes, what protocols do you encourage your staff to follow to keep your rates as low as possible?”
Potential objections
Prevantics Skin Antiseptics: I use alcohol or povidone-iodine to prepare a patient’s skin prior to an injection or a minor surgical procedure.
- Chlorhexidine solution is preferred for skin antisepsis by the CDC.
- All Prevantics® products contain 3.15 percent CHG and 70 percent IPA.
- The Swabstick and Maxi Swabstick are FDA-approved for preoperative indications, pre-activated and ready-to-use with no glass to break and, unlike alcohol or povidone-iodine, provide seven days of continued antimicrobial activity.
- The Swab has a pre-injection indication and an intuitive prep-pad design.
Prevantics Device Swab: I use alcohol prep pads to disinfect needleless access sites before infusing medication in an oncology or rheumatology office.
- The CDC guidelines state, “Some studies have shown that disinfection of the devices with Chlorhexidine/Alcohol solutions appears to be the most effective in reducing colonization.”
- The Prevantics Device Swab contains the first and only 3.15 percent CHG and 70 percent IPA solution authorized by the FDA to disinfect needleless access sites and has a five-second scrub/five-second dry time to help with staff compliance.
Unique to the market, the Prevantics® product portfolio contains PDI’s proprietary formula, 3.15 percent Chlorhexidine Gluconate (CHG) and 70 percent isopropyl alcohol, and is a comprehensive approach to infection prevention for the entire vascular access process.
A wide selection of infection prevention and Prevantics educational materials, including in-service videos, instructions-for-use posters, webinars and whitepapers, are available at www.pdihc.com.
1 Data on file, study report 140304-250
2 CDC HAI Prevalence Survey. Magill, S.S., Edwards, J.R., Bamberg, W., et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N Engl J Med 2014;370:1198-208.