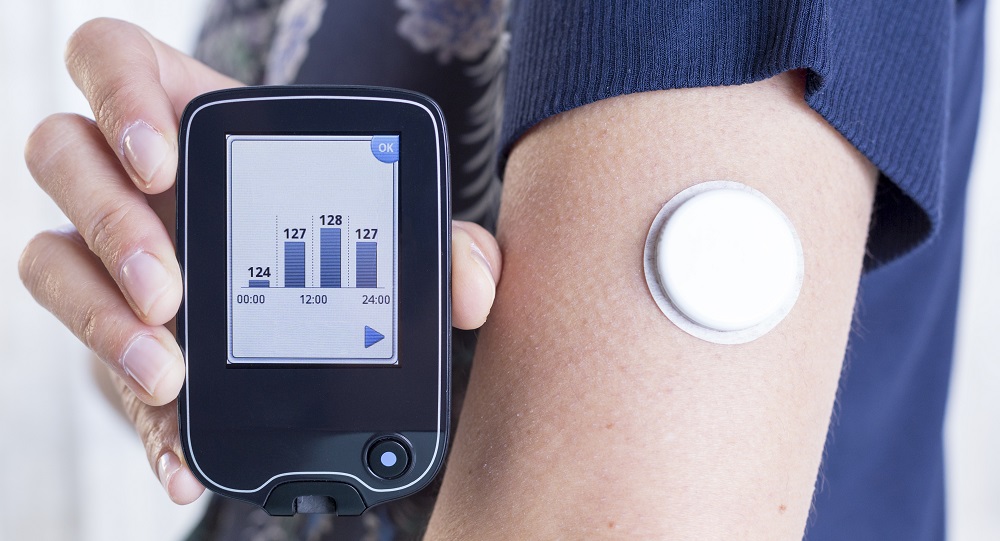
Abbott (Abbott Park, IL) and Tandem Diabetes Care have finalized an agreement to develop and commercialize integrated diabetes solutions that combine Abbott’s continuous glucose monitoring (CGM) technology with Tandem’s insulin delivery systems to provide more options for people to manage their diabetes.
The companies first announced their intention to work together in October 2019. This resulting agreement covers the technical development of device integration and associated commercial support activities.
Through this collaboration, Abbott and Tandem will work to digitally connect their technologies for future automated insulin delivery systems, which will provide people with options to tailor and simplify how they manage their diabetes.
Tandem’s t:slim X2 insulin pump was the first to receive U.S. Food and Drug Administration (FDA) clearance in a new device category, “alternate controller enabled (ACE) infusion pumps,” in 2019.
The special controls for ACE pumps allow for reliable and secure communication with compatible external devices. Abbott’s FreeStyle Libre 2 integrated continuous glucose monitoring (iCGM) system was recently cleared by the FDA for adults and children (4 years and older).
The companies will focus initial commercial activities in the U.S. and Canada with additional geographies considered in the future.