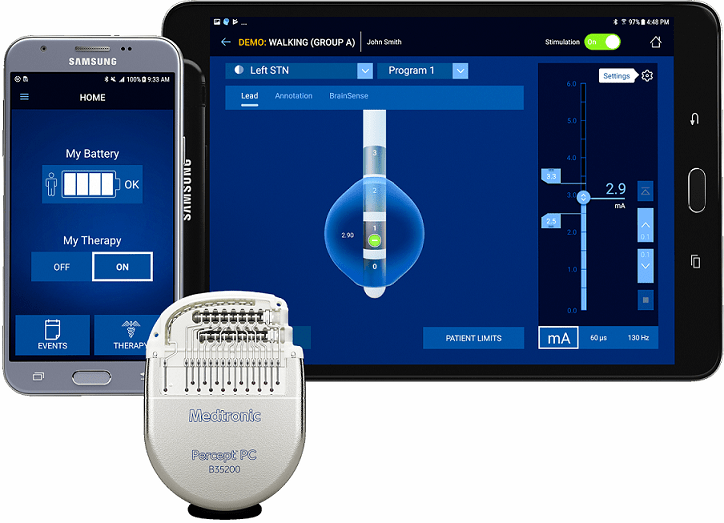
Medtronic plc (Dublin, Ireland) announced it received FDA approval for the Percept PC Deep Brain Stimulation (DBS) system. BrainSense technology makes Percept the first and only DBS neurostimulation system with the ability to chronically capture and record brain signals while delivering therapy to patients with neurologic disorders associated with Parkinson’s disease, essential tremor, dystonia, epilepsy or obsessive-compulsive disorder (OCD), the company said.
DBS is an individualized therapy delivered from a small pacemaker-like device, placed under the skin of the chest or abdomen, to send electrical signals through very thin wires (leads) to a targeted area in the brain related to the symptoms of a neurological disorder, such as Parkinson’s disease.
Physicians can now track patient brain signals and correlate these with patient-recorded actions or experiences, such as symptoms, side-effects, or medication intake, allowing for more personalized, data-driven neurostimulation treatment.