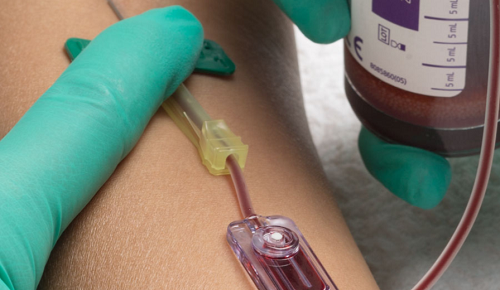
Kurin Inc. (San Diego, CA) was awarded a contract by Intalere (St. Louis, MO) for the Kurin product line. Effective July 1, 2020 the new three-year agreement allows Intalere members to take advantage of special pricing and terms for the FDA 510(k) cleared line of products.
“Kurin’s revolutionary and disruptive approach automatically and passively corrals potential contaminants during blood culture collection,” the company said.
“Contaminated blood cultures are a significant problem, as approximately one-third of the positive results are wrong, exposing these patients to unnecessary antibiotics, extending hospital stays, and impacting larger community health issues, such as antimicrobial resistance and the life-threatening C. diff. infection. Contaminated blood cultures also create a financial burden to hospitals by prolonging hospital stays…”
Kurin Inc., a certified Minority Business Enterprise (MBE), is focused on the design, development, manufacture, marketing, and sale of products that help healthcare providers reduce contaminated blood cultures.