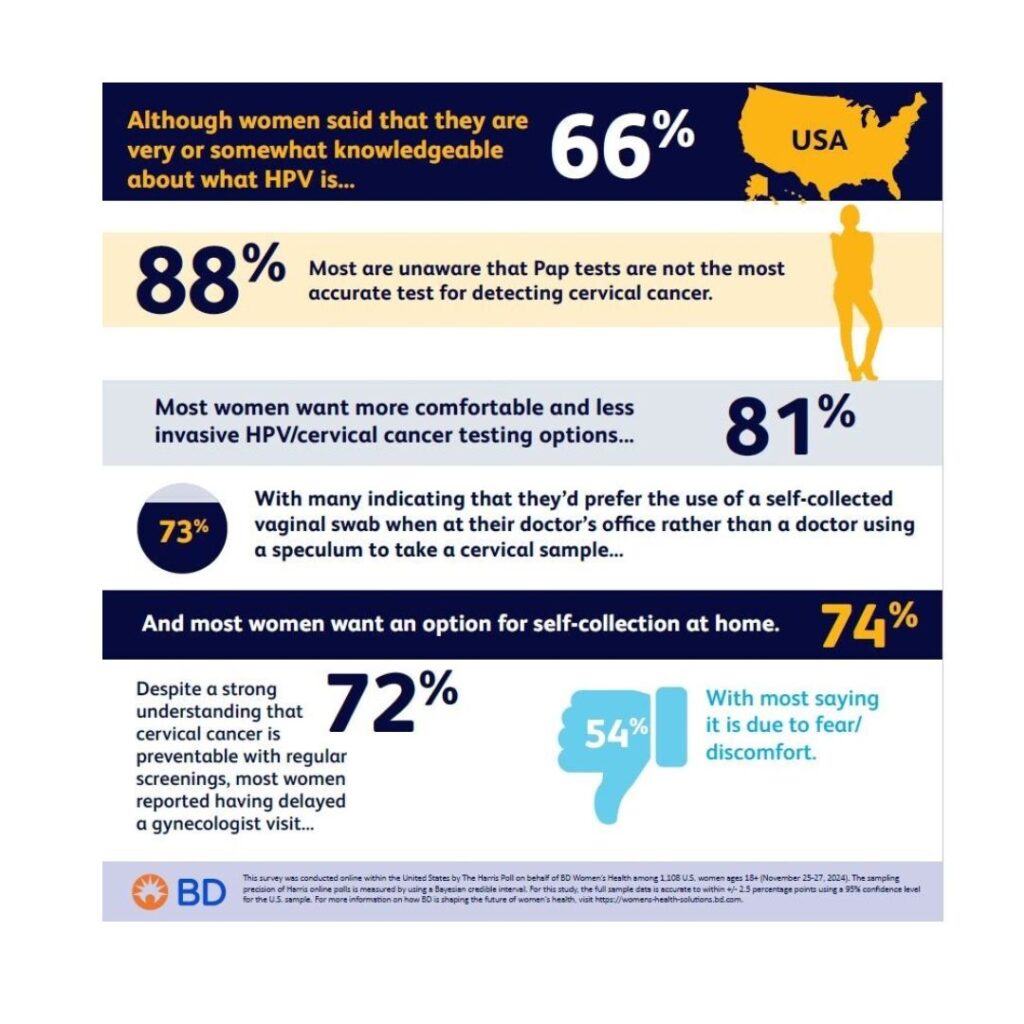
January 9, 2024- BD announced the results of a new survey, revealing that 72% of women in the United States have delayed having a gynecology visit, with many highlighting the need for greater convenience, comfort and ease for this critical cervical cancer screening process.
According to the survey, conducted online by The Harris Poll among over 1,100 adult women in the U.S. in November 2024, despite 62% of women understanding that cervical cancer is preventable with regular screenings, 72% reported having delayed a gynecology visit, with 54% saying it was due to fear or discomfort and 49% citing scheduling-related challenges. Additionally, 50% indicated that they have no idea how often they are supposed to get screened for cervical cancer.
The new study found that 81% of women want more comfortable and less invasive HPV/cervical cancer testing options versus a pelvic exam, with 73% indicating interest in using a self-collection vaginal swab test at the doctor’s office in place of a doctor using a speculum to collect a sample from their cervix. Additionally, 74% of women are interested in having an option for self-collection at home.
The BD Onclarity™ HPV Assay is FDA-approved for self-collection, and it also identifies more individual high-risk types or strains of HPV than any other test. Being able to identify more individual types of HPV means that clinicians can track those types across a patient’s visits to more effectively manage high-risk cases and better guide follow-up for low-risk patients.