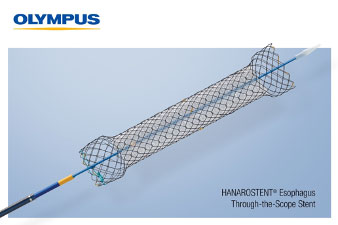
July 17, 2020 – Olympus (Center Valley, PA) has received FDA 510(k) clearance and announced the limited launch of HANAROSTENT Esophagus TTS self-expanding metal stents (SEMS) made by M.I. Tech and now distributed exclusively through Olympus in the U.S.
The company says that the “Through The Scope” (TTS) esophageal stent helps to achieve luminal patency in a variety of clinical applications and is designed for use in palliative treatment of esophageal stricture and/or trachea-esophageal fistula caused by malignant tumors.
The stent offers a fully covered or partially covered option. Pre-loaded into the delivery system, the HANAROSTENT Esophagus TTS fits down a standard therapeutic gastroscope and is deployed for placement in the esophagus.
Unlike over-the-wire esophageal stents, the new 10.5F HANAROSTENT Esophagus TTS allows for accurate and easy esophageal stenting under direct endoscopic visualization…