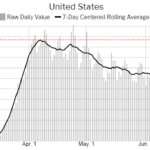
On June 23 and June 24, the U.S. surpassed that high-water mark, at more than 31,700 infections per day. The state of the pandemic in this country has officially passed its previous peak on April 7 of 31,630 cases (or about 10 in every 100,000 Americans testing positive daily). “What is particularly troubling about this trend is that the country as a whole was on the right … [Read more...]
White House to end funding for COVID testing in 7 states amid surge in cases
The Trump administration is ending funding and support for local COVID-19 testing sites around the country this month, as cases and hospitalizations are skyrocketing in many states. The federal government will stop providing money and support for 13 sites across five states which were originally set up in the first months of the pandemic to speed up testing at the local … [Read more...]
Why America’s Healthcare Distributors are the Unsung Heroes of the COVID-19 Crisis
By Scott Adams, Publisher, Repertoire Magazine I recently sat down with two executives from a national distributor to have a candid conversation and give them a voice about the distribution industry’s response to the COVID-19 crisis. Healthcare distribution has been America’s unsung hero during this crisis. The way they worked together to source PPE and move it in record … [Read more...]
Premier, Inc.: Getting Back to Regulatory Readiness
From the Premier, Inc. blog: "COVID-19 interrupted many organizations’ daily operations – including, most likely, a reduced or suspended cadence of regulatory readiness planning. "Any regulatory readiness that’s been on pause, however, will need to pick up quickly. The Joint Commission announced it will begin resuming regular surveys and reviews this month. "Healthcare … [Read more...]
909 Healthcare, Inc. launches CoV19 Prognostic Screening Tool
909 Healthcare, Inc., a client of Carl Meyer and The Wetrich Group, announced the release of the CoV19 prognostic screening tool. CoV19 is a probability assessment that provides early and accurate evaluation of an individual’s likelihood of contracting active COVID-19 and differentiates between influenza and influenza-like illnesses for both symptomatic and asymptomatic … [Read more...]
Stat-Technologies receives Emergency Use Authorization for COVID-19 IgG/IgN rapid test kits
Stat-Technologies (Golden Valley, MN) announced it has received Emergency Use Authorization (EUA) from the U.S. Food & Drug Administration (FDA) for the COVID-19 IgG/IgN rapid test kits it carries. The tests offered by Stat-technologies are manufactured by Healgen Scientific. According to the company, the FDA authorization and Preferred-Provider status allows … [Read more...]
New coronavirus outbreak in Beijing forces city back into lockdown
Beijing has reintroduced strict lockdown measures and is again rolling out mass testing after a new cluster of COVID-19 cases emerged from the city's largest wholesale food market. The Chinese capital reported 36 new Covid-19 cases on Monday, bringing the total number to 79 since a locally transmitted infection was reported on June 12 for the first time in nearly two … [Read more...]
Quidel receives BARDA funding to develop point-of-care diagnostic assay that includes COVID-19
Quidel Corporation (San Diego, CA) announced it has received funding from the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health and Human Services (HHS), to support the development of a point-of-care diagnostic assay that potentially tests for four … [Read more...]
Premier: Meeting Unparalleled Demand for Critical Supplies and Drugs
“While the supply chain has faced isolated spot shortages in the past due to natural disasters and other health crises, no single state, country, government or manufacturer could have fully prepared for the 7X to 30X global surge in demand for critical supplies as a result of COVID-19. “However, over the course of the pandemic, Premier worked with contracted suppliers to … [Read more...]
Quidel receives amended Emergency Authorization for rapid antigen COVID-19 diagnostic assay using Sofia 1 instrument
Quidel Corporation (San Diego, CA) announced it has received an amended Emergency Use Authorization (EUA) from the FDA, allowing Quidel to run its Sofia SARS Antigen FIA, a rapid point-of-care test for COVID-19, on the Sofia Fluorescent Immunoassay Analyzer (Sofia 1), Quidel’s first-generation automated immunoassay instrument. Quidel previously received EUA to run the Sofia … [Read more...]