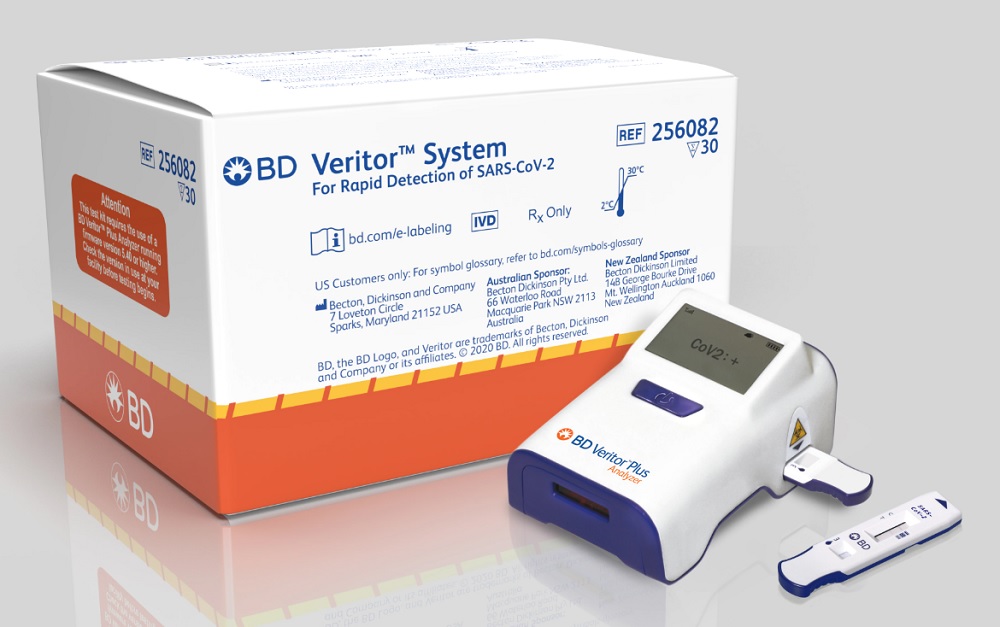
BD (Becton, Dickinson and Company) (Franklin Lakes, NJ) announced that the U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) for a rapid, point-of-care, SARS-CoV-2 diagnostic test for use with the company’s BD Veritor Plus System. This new assay delivers results in 15 minutes on an easy-to-use, highly portable instrument, which enables real-time results and decision making while the patient is still onsite, the company says.
BD is leveraging its global manufacturing network and scale and expects to increase capacity to be able to produce 2 million tests per week by the end of September. The company already expects to produce up to 10 million tests from July through September.
The launch of the BD Veritor Plus System for Rapid Detection of SARS-CoV-2 Assay is the latest effort in the company’s comprehensive response to address critical health needs related to the global pandemic. The new immunoassay test joins a portfolio of three molecular solutions for COVID-19 testing that have been registered for use with the BD MAX Molecular System, including two with EUAs and two with CE mark.
BD intends to pursue 510(k) clearance for the BD Veritor Plus SARS-CoV-2 assay from the FDA at a later time.