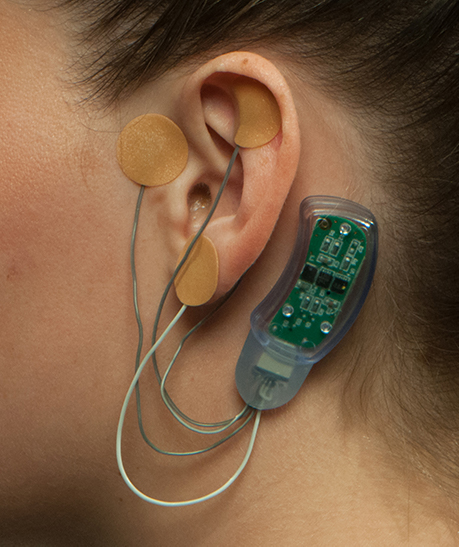
Masimo (Irvine, CA) announced Bridge™, an opioid withdrawal solution that uses neuromodulation to aid in the reduction of symptoms associated with opioid withdrawal. Bridge, which has been granted an FDA De Novo classification, is the first evidence-based, drug-free, non-surgical device of its kind.
In clinical testing, Bridge was found to reduce opioid withdrawal symptoms within 15-30 minutes and provide continuous relief for as long as it was applied, which can be up to 120 hours per device, Masimo says.
The solution consists of a wearable, single-patient-use, percutaneous neurostimulator, fitted behind the ear, which applies gentle electrical impulses to branches of the cranial nerves around the ear. Bridge can be easily applied by a healthcare professional in a simple, non-surgical, in-office procedure, and its use doesn’t interfere with normal daily activities such as work or school…