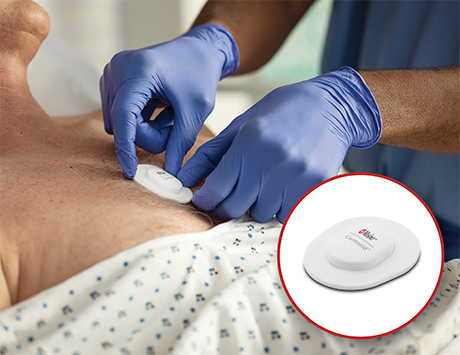
Masimo (Irvine, CA) received FDA clearance for Centroid, a wearable, wireless patient orientation, activity, and respiration rate sensor. According to the company, Centroid helps clinicians monitor patient position to avoid preventable pressure ulcers, and can alert clinicians to sudden movements such as fall-like events. The device also detects chest movements to continuously provide respiration rate, assisting clinicians with additional data that may inform care decisions.
Centroid pairs with the Root Patient Monitoring and Connectivity Platform using Bluetooth to track a patient’s posture, orientation, and activity, providing the ability to monitor patient position and detect changes in position. The data transmitted by Centroid can be displayed in various formats on Root, giving clinicians multiple ways to assess adherence to protocols regarding tissue stress and to tailor care to the specific needs of each patient. The company says that the device’s respiration rate performance is accurate to within 3 respirations per minute (rpm) in the range of 8 to 35 rpm.
Centroid is indicated for the orientation monitoring of patients who may be susceptible to pressure ulcers, by tracking patient movement and activity and can provide alerts based on the duration in a static position to help clinicians adhere to hospital patient turn protocols…