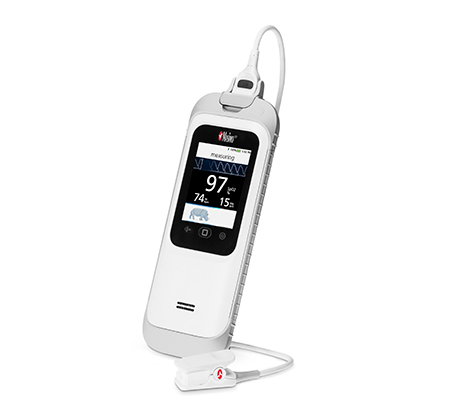
Masimo (Irvine, CA) announced the results of a study published in Pneumonia in which independent researchers in New Delhi, India, investigated the accuracy of Masimo RRp on pediatric patients with the Rad-G Pulse Oximeter by comparing it to clinician-determined values while performing routine assessment of children admitted to outpatient and emergency departments.
The researchers concluded, that “there is a high degree of agreement between pleth-based RR using a [pulse oximetry device] and physician measured RR, indicating that the former provides reliable and accurate measurement.”
Masimo RRp is CE marked and U.S. FDA 510(k) cleared. Rad-G is currently CE marked and the U.S. FDA 510(k) clearance is pending.