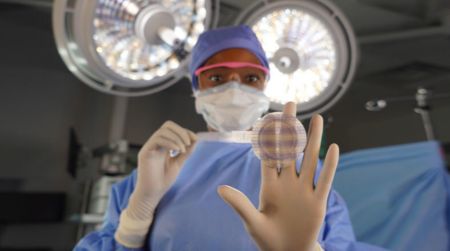
April 24, 2025- BD announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) and the commercial launch of Phasix™ ST Umbilical Hernia Patch, the first and only fully absorbable hernia patch on the market designed specifically for umbilical hernias.
Composed of Poly-4-hydroxybutyrate (P4HB), a biologically-derived material, with the added benefits of a proven hydrogel barrier based on Sepra® Technology, Phasix™ ST Umbilical Hernia Patch offers a unique absorbable solution to umbilical hernia repair. It can be deployed using the same technique surgeons use with a traditional permanent mesh patch, while providing material optionality.