February 26, 2021 – B. Braun Medical Inc. (Bethlehem, PA) announced it has received approval from the U.S. Food & Drug Administration (FDA) for the first and only Acetaminophen Injection available in multiple doses.
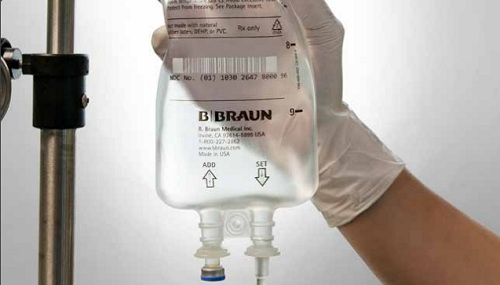
Acetaminophen Injection, manufactured by B. Braun in Irvine, California, is available in our sturdy, flexible PAB IV Bags in both 1,000 mg in 100 mL and 500 mg in 50 mL doses.
The company’s PAB IV bags are not made with PVC, DEHP or latex.
B. Braun said its Acetaminophen Injection is manufactured using dual-sourced Active Pharmaceutical Ingredients (API) to mitigate potential supply risks.
”Acetaminophen Injection is the first of multiple injectable drugs B. Braun plans to launch in the coming years to help meet the needs of patients for medications in high demand,” said Leigh Nickens, Director of Marketing, Fluid Therapy & Injectable Drugs “It represents our commitment to improve the security of supply by manufacturing pharmaceuticals in the United States at our newly expanded and modernized facility in Irvine, California.”