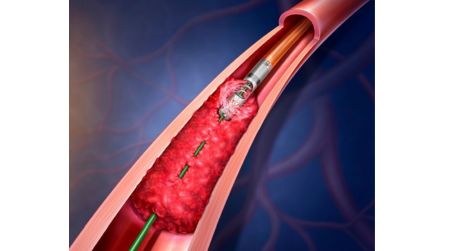
May 28, 2025- BD announced plans to initiate a patient data registry for the Rotarex™ Atherectomy System to measure real-world outcomes for patients with peripheral artery disease (PAD).
Known as “XTRACT,” this prospective, multi-center, single-arm, post-market registry study will assess the clinical performance of the Rotarex™ Atherectomy System in the treatment of U.S. patients with PAD lesions. The XTRACT Registry is being led in partnership with Co-Principal Investigators, Dr. Prakash Krishnan, an interventional cardiologist, and Dr. Todd Berland, a vascular surgeon. The registry will enroll up to 600 patients at approximately 100 clinical sites across the United States, with the first patient enrollment expected later this year. Clinical follow-up evaluations will occur after 30 days, 6 months and 12 months post-procedure to assess safety and effectiveness of outcomes.