Repertoire Magazine – June 2021
Hand hygiene is the primary measure for providing safer care in healthcare facilities1. Hand hygiene in the healthcare environment consists of a combination of washing hands with soap and water and using an Alcohol-based hand rub (ABHR). According to nationally accepted guidelines from the Centers for Disease Control and Prevention (CDC), the majority of hand hygiene events should be performed using an ABHR.
The CDC guidelines for hand hygiene in healthcare settings recommend seeking feedback from healthcare workers (HCW) regarding their hand hygiene product’s overall acceptability (skin tolerance, feel and fragrance) in order to maximize product acceptance. Proper hand hygiene is more than just washing and sanitizing regularly.
Product acceptability is an important driver of hand hygiene compliance; that is; if HCW do not like the product, they may be less likely to use it, consequently affecting hand hygiene compliance. Published studies support the concept that as skin irritation increases, hand hygiene compliance decreases.2,3 Human factor elements oftentimes do not get the attention that they warrant. While a major emphasis in the selection process for an ABHR product is efficacy, an important determinant of hand hygiene compliance is acceptance of the products by HCWs.5
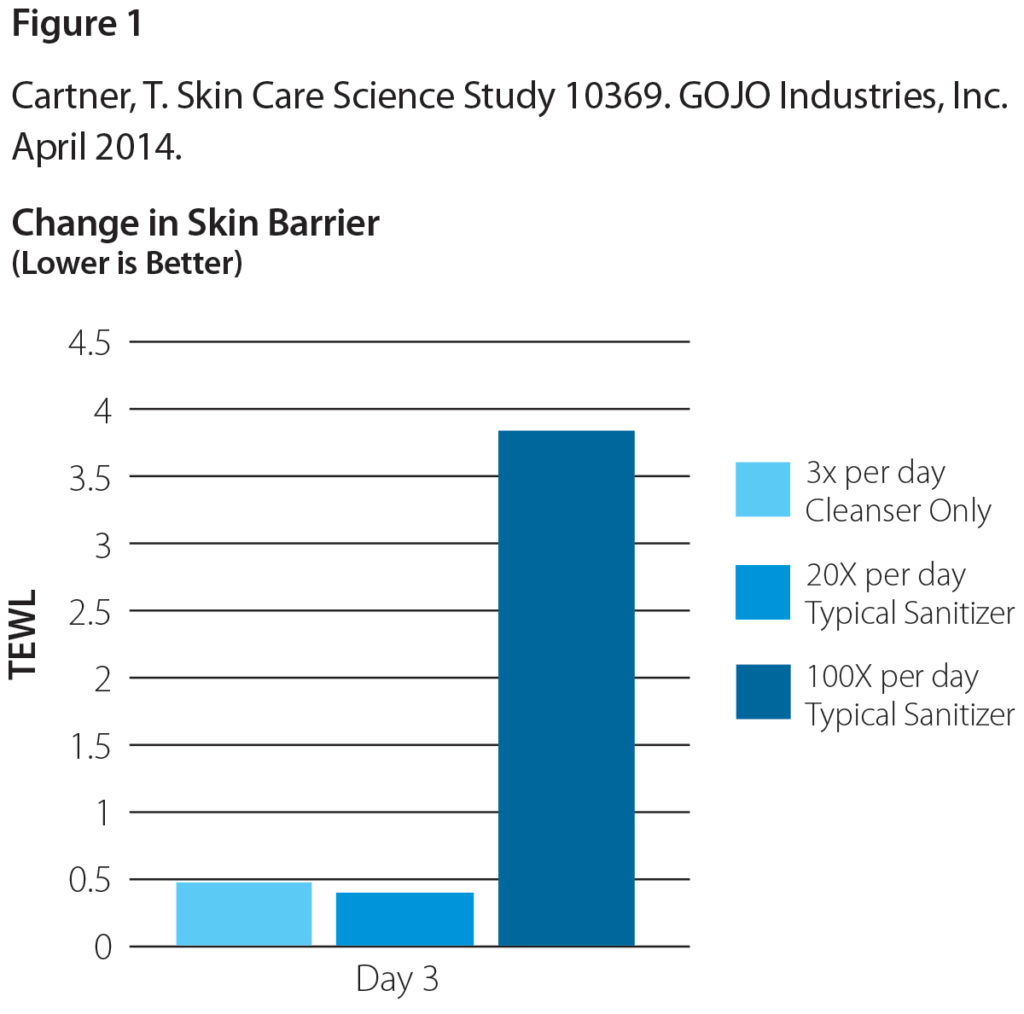
One study found that a typical sanitizer used at 20 times per day was no more damaging than handwashing with soap and water alone.4 However, once hand sanitizing events increased 5-fold, there was significantly more changes in skin barrier as measured by transepidermal water loss (TEWL). In very high compliance environments, i.e., hand hygiene events in excess of 100 times per day, it is important to select product formulations better suited to meet the skin health needs of HCW. Figure 1 demonstrates the effects of handwashing versus hand sanitizing regimens on skin by evaluating changes in skin barrier, as measured TEWL.
As you can see, if poorly formulated hand hygiene products are selected, it could have an adverse impact on HCW acceptance, skin condition, and hand hygiene compliance, which is why some ABHR contain emollients and moisturizing ingredients to counteract this effect. Fragrances, gelling agents, foaming agents, and other ingredients are also added to improve product aesthetics.
Since higher levels of alcohol tend to reduce ABHR aesthetics, it is important to choose a product with no tradeoff between efficacy and product acceptance. Once above the recommended 60% alcohol threshold, the total ABHR formulation has a much larger impact on antimicrobial efficacy than alcohol level. The unique blend of non-active ingredients can either enhance or hinder the ability of alcohol to inactivate microorganisms on the skin and play a significant role in the ability of ABHR formulations to meet efficacy standards. Beyond efficacy, product formulation determines other critical aspects such as skin tolerability and aesthetics, which directly affect product acceptance and usage rates, and indirectly affect clinical outcomes.
The human factor variables of hand hygiene compliance such as product use decisions and patterns of the healthcare worker are complex and must be considered as part of the equation. Even well formulated hand hygiene products will be inherently limited in their ability to reduce germs by their pattern of use in the healthcare environment. Thus, the “best” products are those that meet the required efficacy while optimizing product acceptance elements to ensure maximum product usage.
Fortunately, newer ABHR formulation technology can achieve a balance of properties that enhance the user experience and reduce barriers to use, while exhibiting superior antimicrobial efficacy. Choosing products that are formulated with mild and effective ingredients offers HCW protection against both pathogens and skin damage, which is a critical foundation for a hand hygiene program.