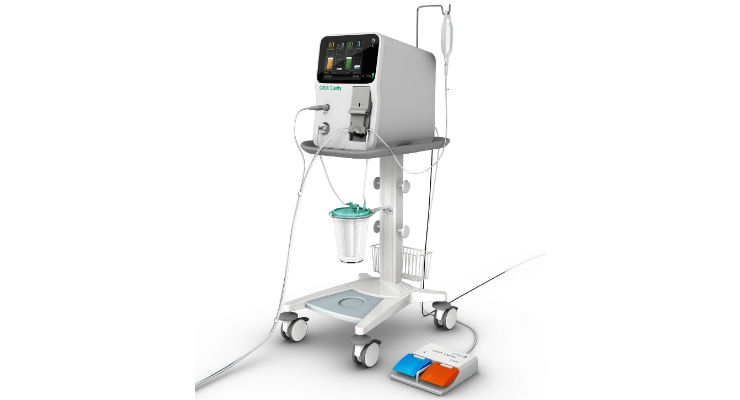
July 22, 2020 – Integra LifeSciences Holdings Corporation (Princeton, NJ) received FDA clearance for a specific indication for neurosurgery for its CUSA Clarity Ultrasonic Surgical Aspirator System.
Launched in 2017, the CUSA Clarity is the latest addition to the CUSA ultrasonic tissue ablation lineup.
Although the CUSA system has been used for over 40 years in neurosurgical applications, this specific neurosurgery indication shows CUSA Clarity can be used safely and effectively in neurosurgery for resection of tumors ranging from soft to firm consistencies, which includes removal of primary and secondary malignant, and benign brain and spinal tumors, including meningiomas and gliomas.